What is the molar mass of aspartic acid c4o4h7n – Unveiling the Molar Mass of Aspartic Acid (C4O4H7N): Delving into the intricacies of its molecular composition and significance in biological processes, this discourse provides a comprehensive understanding of this essential amino acid.
Aspartic acid, a crucial component of proteins, plays a vital role in cellular metabolism and neurotransmission. Its molar mass, a fundamental property, determines its reactivity and behavior in various chemical and biological systems.
1. Definition of Molar Mass
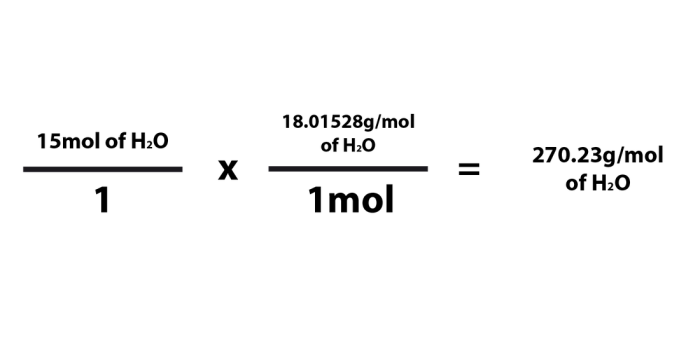
Molar mass is a fundamental concept in chemistry that represents the mass of one mole of a substance. It is a measure of the average mass of all the atoms or molecules in a given compound. The molar mass is expressed in grams per mole (g/mol) and is a crucial parameter for determining the amount of a substance present in a given sample.
The molar mass is closely related to the molecular weight of a compound. Molecular weight refers to the mass of a single molecule of a substance, while molar mass represents the mass of one mole of that substance, which is equal to 6.022 × 10 23molecules.
2. Composition of Aspartic Acid: What Is The Molar Mass Of Aspartic Acid C4o4h7n
Aspartic acid, also known as aspartate, is an amino acid with the chemical formula C 4O 4H 7N. It is a non-essential amino acid, meaning that it can be synthesized by the body and is not required to be obtained from the diet.
Aspartic acid is composed of the following elements:
- Carbon (C): 4 atoms
- Oxygen (O): 4 atoms
- Hydrogen (H): 7 atoms
- Nitrogen (N): 1 atom
The atomic masses of these elements are:
- Carbon: 12.011 g/mol
- Oxygen: 15.999 g/mol
- Hydrogen: 1.008 g/mol
- Nitrogen: 14.007 g/mol
3. Calculation of Molar Mass
To calculate the molar mass of aspartic acid, we multiply the atomic mass of each element by the number of atoms of that element in the chemical formula and then add the results:
Molar mass = (4 × 12.011 g/mol) + (4 × 15.999 g/mol) + (7 × 1.008 g/mol) + (1 × 14.007 g/mol)
Molar mass = 133.103 g/mol
Therefore, the molar mass of aspartic acid is 133.103 g/mol.
4. Applications of Aspartic Acid
Aspartic acid is an important amino acid involved in various biological processes:
- Protein synthesis:Aspartic acid is one of the 20 amino acids that are used to build proteins. It is essential for the synthesis of proteins involved in muscle growth, tissue repair, and immune function.
- Metabolism:Aspartic acid plays a role in the citric acid cycle, which is a central metabolic pathway that generates energy for cells. It also participates in the synthesis of other amino acids and nucleotides.
FAQ Compilation
What is the chemical formula of aspartic acid?
C4O4H7N
How many carbon atoms are present in aspartic acid?
4
What is the molecular weight of aspartic acid?
133.1 g/mol