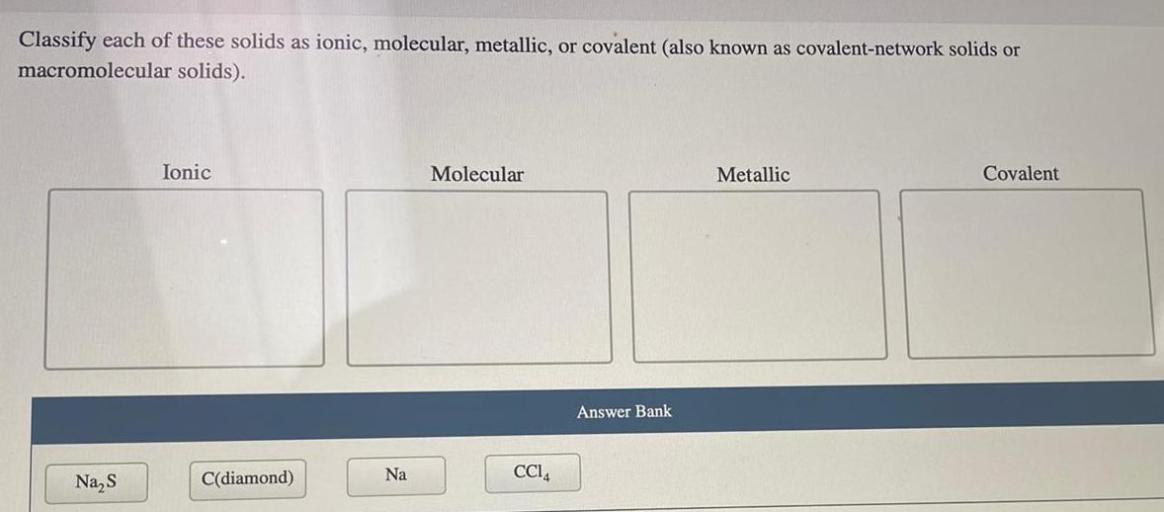
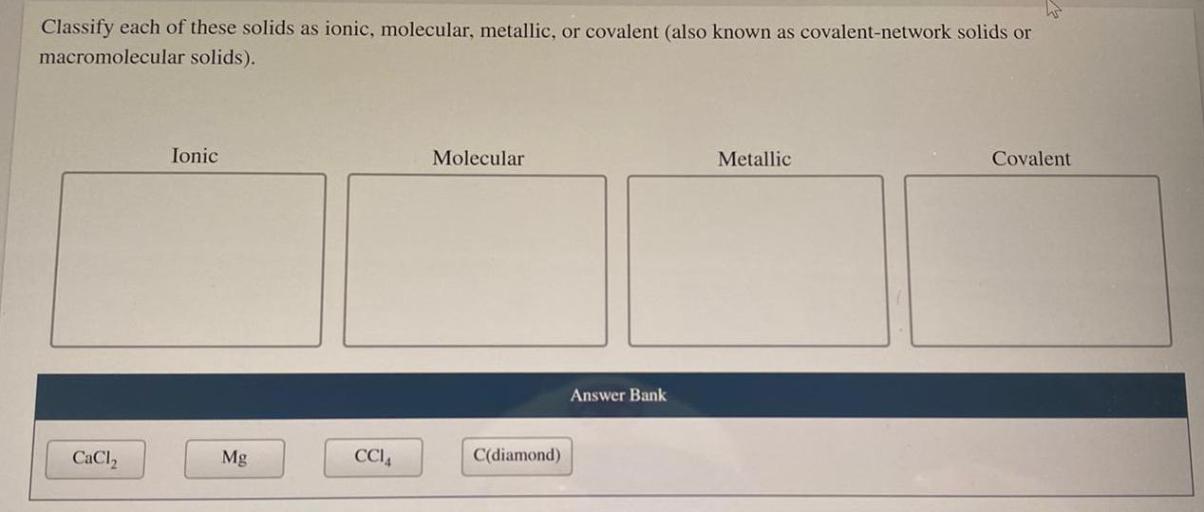
Classify each of these solids as ionic molecular or metallic. The classification of solids is a fundamental aspect of chemistry, as it provides insights into their physical and chemical properties. This guide will delve into the key characteristics that distinguish ionic, molecular, and metallic solids, presenting a step-by-step approach to their classification.
Understanding the differences between these three types of solids is crucial for comprehending their behavior in various applications, ranging from materials science to chemical engineering.
Introduction: Classify Each Of These Solids As Ionic Molecular Or Metallic
The classification of solids is a fundamental aspect of chemistry and materials science. It provides insights into the physical and chemical properties of solids, which are essential for understanding their behavior and applications. Solids can be classified into three main categories: ionic, molecular, and metallic, each with distinct characteristics.
Properties of Ionic, Molecular, and Metallic Solids
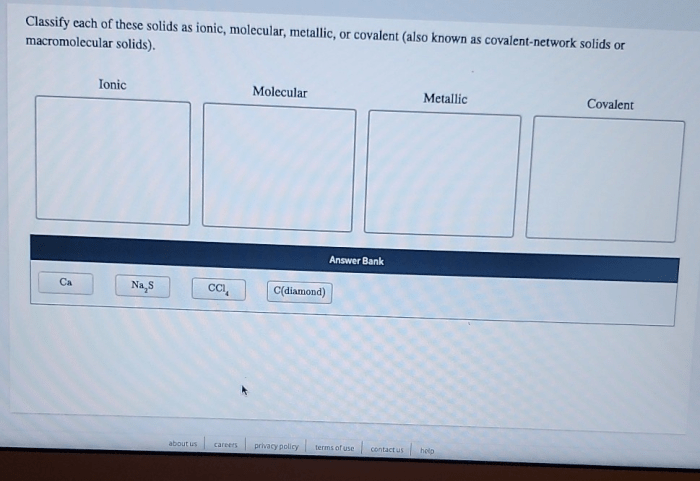
Ionic solids are composed of positively charged ions (cations) and negatively charged ions (anions). They are typically hard, brittle, and have high melting points. Examples of ionic solids include sodium chloride (NaCl) and potassium chloride (KCl).
Molecular solids are composed of neutral molecules. They are typically soft, have low melting points, and are often volatile. Examples of molecular solids include sugar (C12H22O11) and ice (H2O).
Metallic solids are composed of positively charged metal ions surrounded by a sea of mobile electrons. They are typically shiny, malleable, ductile, and have high electrical and thermal conductivity. Examples of metallic solids include copper (Cu), aluminum (Al), and iron (Fe).
Classification of Solids
The classification of solids is based on their chemical composition and bonding characteristics. The following criteria are used to classify solids:
- The presence or absence of ions
- The type of chemical bond (ionic, covalent, or metallic)
- The arrangement of atoms, molecules, or ions in the solid
The following step-by-step procedure can be used to classify solids:
- Determine if the solid contains ions. If it does, it is an ionic solid.
- If the solid does not contain ions, determine if it is composed of neutral molecules. If it is, it is a molecular solid.
- If the solid is not composed of neutral molecules, it is a metallic solid.
Examples of Solids
| Ionic | Molecular | Metallic | Examples |
|---|---|---|---|
| Sodium chloride (NaCl) | Sugar (C12H22O11) | Copper (Cu) | |
| Potassium chloride (KCl) | Ice (H2O) | Aluminum (Al) | |
| Calcium carbonate (CaCO3) | Dry ice (CO2) | Iron (Fe) | |
| Magnesium oxide (MgO) | Naphthalene (C10H8) | Gold (Au) |
Applications of Solid Classification
The classification of solids has numerous practical applications in materials science and chemical engineering. Some examples include:
- Materials science:The classification of solids helps in the design and development of new materials with specific properties. For example, ionic solids are often used as electrolytes in batteries, while metallic solids are used in electrical conductors.
- Chemical engineering:The classification of solids is essential for understanding the behavior of solids in chemical processes. For example, the solubility of ionic solids in water is important for designing crystallization processes.
Questions and Answers
What are the key properties that distinguish ionic, molecular, and metallic solids?
Ionic solids are characterized by strong electrostatic forces between oppositely charged ions, molecular solids are held together by weak intermolecular forces, and metallic solids are characterized by a sea of mobile electrons.
How can I classify a solid as ionic, molecular, or metallic?
To classify a solid, consider its physical properties, such as electrical conductivity, solubility, and melting point. Ionic solids are typically hard, brittle, and have high melting points, molecular solids are often soft, have low melting points, and are poor conductors of electricity, and metallic solids are typically shiny, malleable, and good conductors of electricity.